Key Features
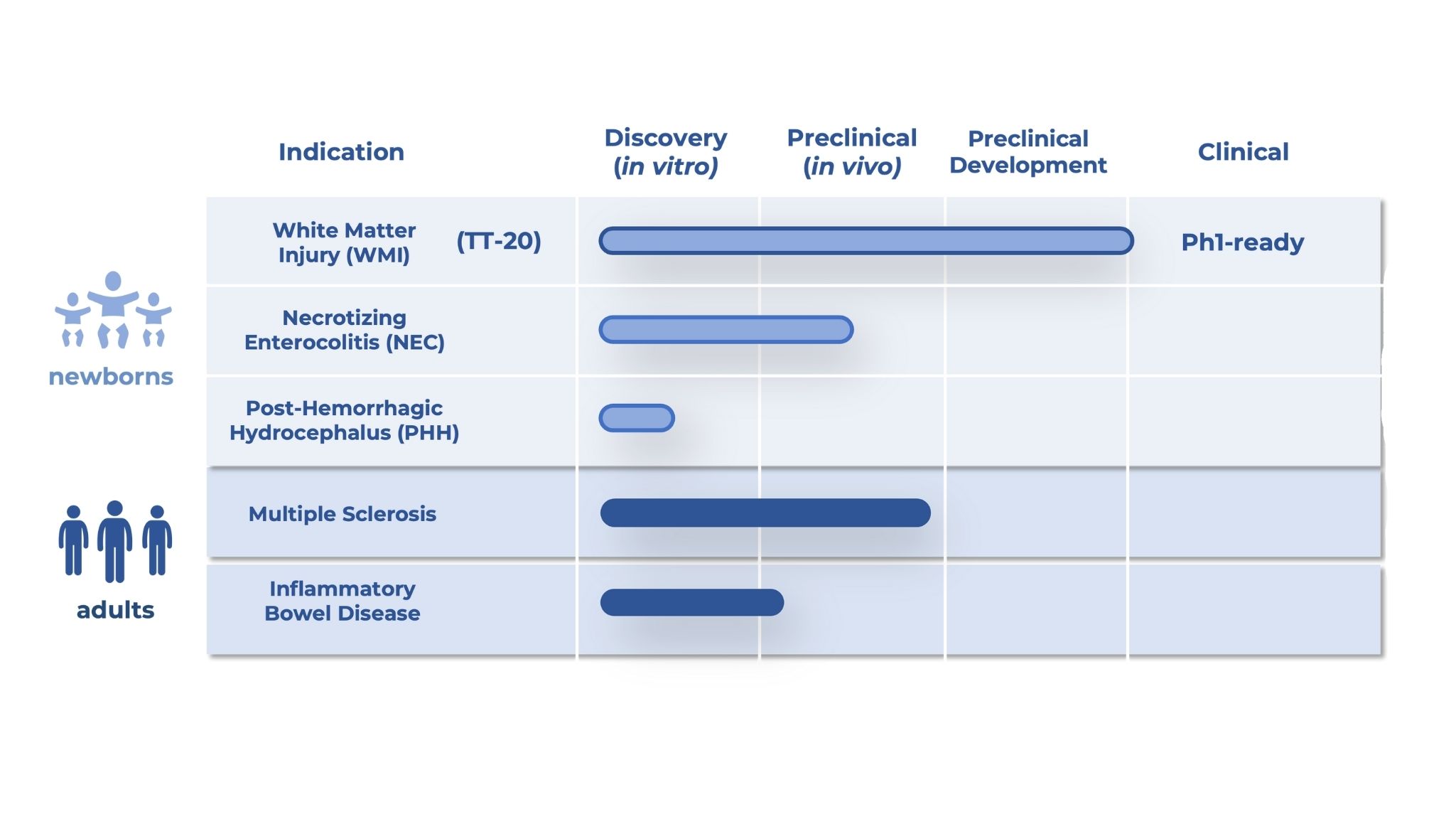
Lead Asset Progressing to Clinic
The IND-enabling program for our lead asset, TT-20, has been agreed upon with the United States FDA, and approved for a first-in-human trial by the Australian Human Research Ethics Committee (HREC) .
Criticial Patient Need, No Other Option
TT-20 represents the world’s first potential treatment for neonatal White Matter Injury (WMI), and is poised to establish a leading position within the underdeveloped Neonatal Intensive Care Unit (NICU) therapeutics market.
Starting at Birth, Scaling Across Lifespan
The biological mechanisms driving our neonatal programs translate directly to adult neuro-regenerative and anti-inflammatory conditions, establishing a development platform with multi-generational reach.