The Market Opportunity: Addressing a Critical Unmet Need
Tellus Therapeutics is targeting one of neonatology’s most pressing challenges—a condition that affects thousands of newborns annually and leaves families facing decades of medical, therapeutic, and personal costs.
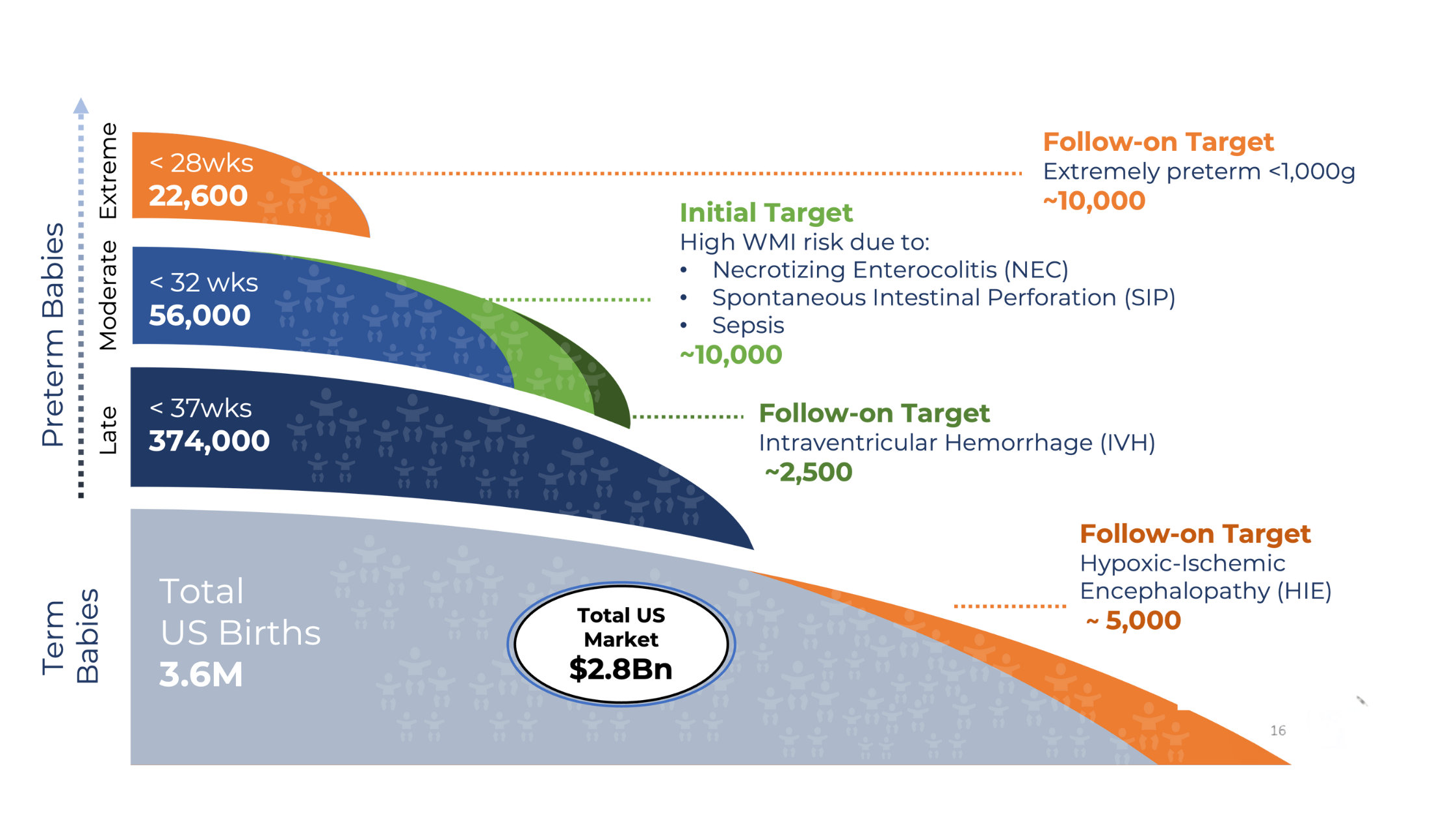
White matter injury (WMI) is the most common brain injury in preterm infants, affecting babies born before 32 weeks gestational age. Caused by hypoxia or inflammation from conditions like necrotizing enterocolitis and sepsis, WMI results in the loss of critical oligodendrocyte progenitor cells, leading to incomplete myelination of the developing brain.
This translates to neurodevelopmental impairment (NDI)—a spectrum of lifelong motor and cognitive deficits including cerebral palsy, detectable as early as 3 months of age and persisting throughout the patient’s entire life. With approximately 10,000 high-risk infants affected annually in the US alone, and no approved treatments currently available, WMI represents both a devastating clinical need and a significant commercial opportunity.
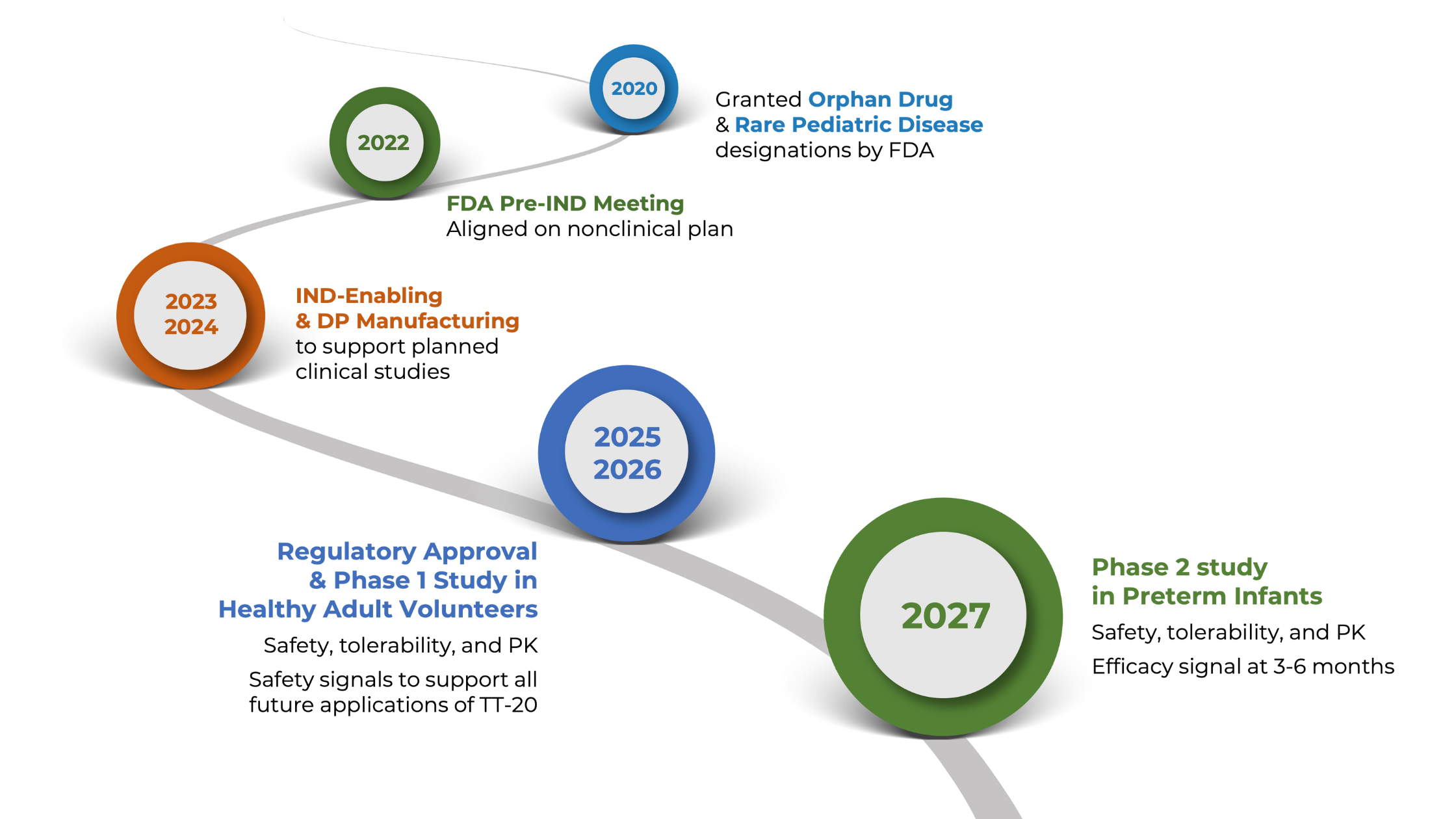
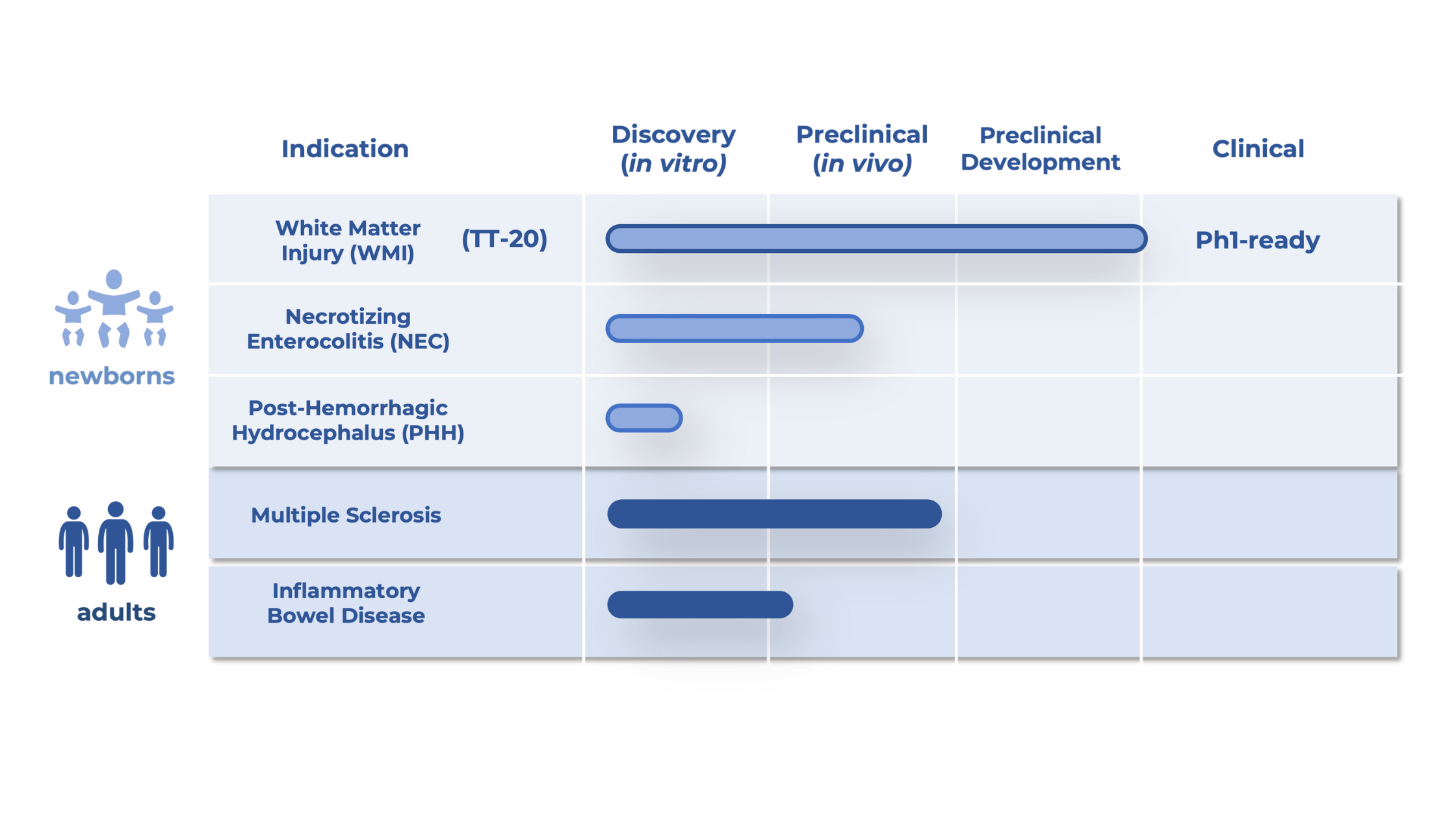
Tellus is uniquely positioned to capture this market with TT-20, our lead asset now advancing toward clinical trials, in a largely unexplored NICU therapeutics space valued at over $2.8B, with potential expansion to adult indications.